Quantum Mechanics Introduction
— 1. Motivation —
— 1.1. New results, new conceptions —
- In principle the disturbance can, in some cases, be made as small as one wants. The fact that exists a limit in our measuring apparatus lies in the nature of the apparatus and not in the nature of the theory being used.
- There exists some disturbances whose effect can't be neglected. Nevertheless one can always calculate exactly the value of the effect of the disturbance and compensate it in the value of the quantity that is being measured afterwards.
- Black body radiation.
- Photoelectric effect.
- Rydberg–Ritz combination principle.
- The existence and stability of atoms.
- Stern-Gerlach's experiment.
- Electron diffraction.
- ...
- Corpuscular entities were demonstrating wavelike behavior.
- Wave entities were demonstrating pointlike behavior.
- There exists a statistical nature in atomic and subatomic phenomena that seems to be an essential characteristic of Nature.
- Nature's atomic nature (no pun intended) implies that the process of measure has to be thought over: some disturbances can't be made arbitrarily small since there exists a natural limit to how small a disturbance can be made.
— 1.2. Double-slit experiment —
— 1.2.1. Double-slit and particles —
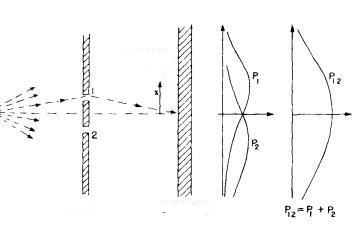
— 1.2.2. Double-slit and waves —
Now if we direct a beam of waves on the same experimental apparatus the pattern one observes in the second wall is:
Now what we'll study is the intensity curve. The intensity curve $ {I_1}$ is the result one would observe if only slit $ {1}$ was open, while the intensity curve $ {I_2}$ is what one would observe if only slit $ {2}$ was open. The resulting intensity is given by the mathematical expression $ {I_{12}=|h_1+h_2|^2= I_1+2I_1I_2 \cos \theta}$. The last term is responsible for the interaction between the waves that come from slit $ {1}$ with the waves that come from slit $ {2}$ which ultimately is what causes the interference pattern one observes in the second wall.
— 1.2.3. Double slit and electrons —
Now that we familiarized ourselves with the behavior of particles and waves under the double slit we'll study what one observes when we subject an electron beam through the same apparatus.
Experimental results show conclusively that electrons are particles so we're expecting to see the same pattern that we saw for macroscopic particles.
Nevertheless this is what Nature has for us!: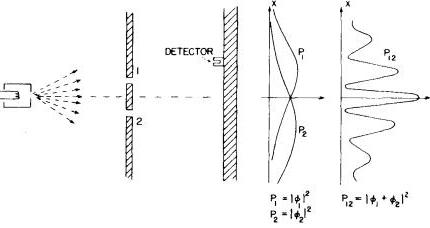
In order to explain what we're observing one has to assume that each probability curve $ {P_i}$ is associated to a probability amplitude $ {\phi_i}$. In order to calculate the probability one has to calculate the modulus squared of the probability amplitude, $ {P_i=\phi_i^2}$. Thus one has to first calculate the sum of probability amplitudes of an electron passing through slit $ {1}$ or passing through slit $ {2}$ and only then should we calculate the modulus squared of the probability amplitude of an electron passing through slit $ {1}$ or slit $ {2}$:
$ \displaystyle P_{12}=|\phi_1+\phi_2|^2$
— 2. Basic concepts and preliminary definitions —
After our initial discussion I suppose that you understand why physicists had the need to introduce a new paradigm that made sense of what's happening in the atomic and sub- atomic level. Now it's time to introduce our usual initial definitions.
| Definition 1 An operator is a mathematical operations that carries a given function into another function. |
As an example of an operator we have $ {\dfrac{d}{dx}}$ which a transforms a functions into its $ {x}$ derivative. As another example of an operator we have $ {2\times}$ that transforms a function into its double.
| Definition 2 The linear momentum is represented by the operator
$ \displaystyle p=\dfrac{\hbar}{i}\dfrac{d}{dx} \ \ \ \ \ (1)$
|
| Definition 3 The energy of a particle is represented by the operator:
$ \displaystyle E=i\hbar\dfrac{d}{dt} \ \ \ \ \ (2)$
|
| Definition 4 For a free particle the following mathematical relationships are valid:$ {\begin{aligned} \label{eq:relacoesdebroglie} k &= \frac{\hbar}{p}\\ \omega &= \frac{E}{\hbar} \end{aligned}}$ |
— 3. Quantum Mechanics Axioms —
After this batch of initial definitions that allows us to know the entities that will take part in the construction of our quantum vision of the world it is time for us to define the rules that govern them
| Axiom 1 The quantum state is defined by the specification of the relevant physical quantities and is represented by a complex function $ {\Psi(x,t)}$ |
| Axiom 2 To every observable in Classical Physics ($ {A}$) there corresponds a linear, Hermitian operator ($ {\hat{A}}$) in quantum mechanics. |
| Axiom 4 The probability that a particle is in the volume element $ {dx}$ is denoted by$ {P(x)dx}$ and is calculated by
$ \displaystyle P(x)dx=|\Psi(x,t)|^2dx \ \ \ \ \ (4)$
|
| Axiom 5 The average value of a physical quantity $ {A}$, represented by $ {<A>}$, is
$ \displaystyle <A> = \int \Psi^*\hat{A}\Psi \ \ \ \ \ (5)$
|
Where the integral is calculated in the relevant volume region.
| Axiom 6 The state of a quantum system evolves according to the Schrodinger Equation.
$ \displaystyle \hat{H}\Psi= i\hbar\frac{\partial \Psi}{\partial t} \ \ \ \ \ (6)$
|
Where$ {\hat{H}}$ is the hamiltonian operator and corresponds to the total energy of a quantum system.

0 comments:
Post a Comment